Rare earth elements (REE) are a group of 17 chemical elements vital to today’s technology and the clean energy revolution. These include neodymium (Nd), cerium (Ce), lanthanum (La), europium (Eu) and dysprosium (Dy). While not truly rare in Earth’s crust, these elements are seldom found in concentrated, economically extractable deposits, making their extraction complex, expensive and strategically important.
China dominates rare earth refining, providing the vast majority of the world’s supply. The United States heavily relies on these imports.
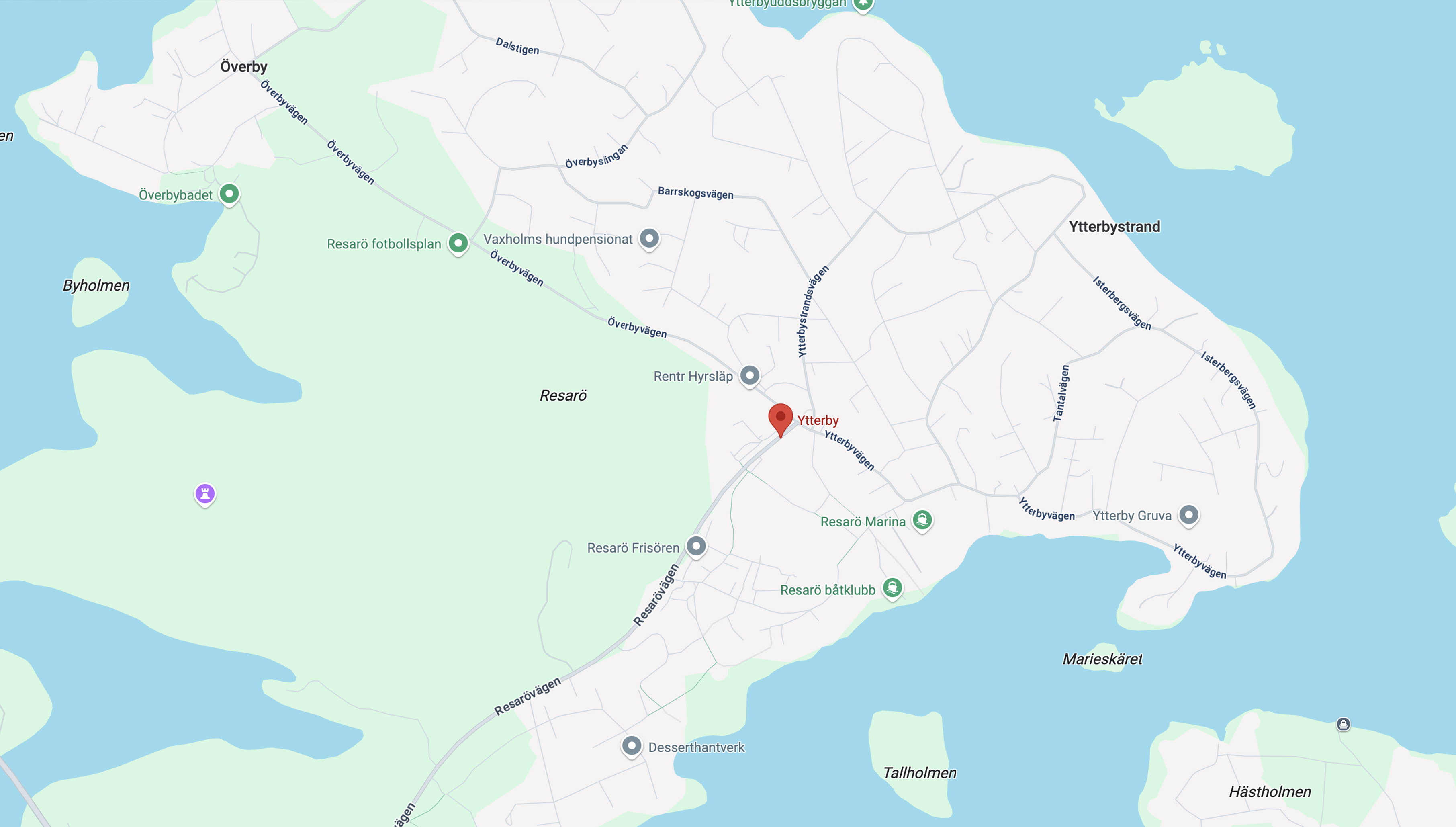
The discovery of rare earths began in the late 18th century in Ytterby, Sweden. In 1787, Carl Axel Arrhenius, an army officer and mineralogist, discovered a mysterious black mineral in a local quarry mined for feldspar used to make porcelain and glass. Chemistry professor Johan Gadolin analyzed this mineral in 1794 and discovered a new “earth,” later named yttrium (Y), the first rare earth element ever identified. Gadolin’s pioneering work laid the foundation for isolating other elements such as terbium (Tb), erbium (Er) and ytterbium (Yb), all named after the small settlement of Ytterby, located on the Island of Resarö near Stockholm.
Rare earth minerals are not pure elements, but are found in complex minerals like bastnäsite [(Ce, La, Y)CO₃F], monazite [(Ce, La, Nd, Th)PO₄] and xenotime (YPO₄), where rare earths are chemically bound to oxygen, phosphate or carbonate ions.
Extraction and Refining Process ⚙️

Extracting rare earth elements is a complicated, multi-step process that is environmentally challenging due to toxic waste ☠️ and radioactive byproducts ☢️, demanding careful management:
- Mining: Ore is mined from deposits containing 1–10% rare earth minerals
- Crushing & Grinding: Ore is crushed and ground to liberate minerals
- Physical Separation: Magnetic or gravity techniques concentrate rare earth minerals
- Chemical Processing: Acids or alkalis dissolve the minerals, releasing rare earth ions
- Separation: Solvent extraction or ion exchange isolates individual elements
- Precipitation & Drying: Purified elements are precipitated as oxides or carbonates
- Metal Production: Further refining produces metals or alloys used in industry
Rare earth elements power everyday devices such as smartphones 📱, computers 💻, flat-screen TVs, fluorescent and LED lights, headphones, electric vehicles, wind turbines, MRI machines 🏥, hard drives, catalytic converters and military technologies like jet engines and missile guidance systems.
Real-World Examples of Rare Earth Use
- NVIDIA and Electronics: NVIDIA’s GPUs power everything from gaming computers 🎮 to AI data centers. While NVIDIA does not directly handle rare earths, many components in their devices rely on them. Neodymium and dysprosium magnets are used in cooling fans for GPUs and servers, while europium and terbium enhance display screens that showcase NVIDIA’s graphics
- Missile Defense Systems: Rare earth elements such as neodymium and dysprosium are integral to the strong permanent magnets found in missile guidance systems and advanced radar technology 📡. These magnets provide precise control for steering missiles and stable signal processing in radar, ensuring accuracy and reliability in defense applications. Additionally, rare earth-based materials improve the efficiency and durability of jet engines ✈️ powering military aircraft
- Car Manufacturing: In electric and hybrid vehicles 🚙⚡, neodymium and dysprosium are key components in the permanent magnets of electric motors, enabling powerful and efficient propulsion. Lanthanum and cerium are critical in nickel-metal hydride (NiMH) batteries used in hybrids and catalytic converters that reduce harmful emissions 🚦 in combustion engines. These rare earths help automakers meet stricter environmental standards while advancing vehicle performance.
What If Rare Earth Minerals Were Gone? ⚠️
If rare earth minerals suddenly became unavailable or severely limited, the impact on technology, industry and daily life would be profound and wide-ranging.
Rare earth minerals might be hidden deep in the Earth, but their impact is anything but small. As the backbone of clean energy, advanced technology and modern life, these elements deserve our attention - not just for their scientific value, but for the future they help build. As we move forward, understanding where they come from, how we use them and the challenges around their supply will be crucial for anyone who cares about growth, innovation and sustainability. Keep your eyes on rare earths - they’re quietly shaping the world ahead.
Now that you’ve read today’s blog, why not take what you’ve learned and play today’s matching quiz on your Quizefy app? Many of the answers can be found right here. We publish an on-trend, hint-filled blog at www.quizefy.com every Tuesday, along with a matching quiz in your Quizefy app. We think they’re a great combination and a great way to Strut Your Smart.

